COVID-19 vaccine is safe during pregnancy and may also protect baby, study shows
Not only have COVID-19 vaccines been found to be safe for pregnant women, but a new study by Northwestern Medicine says expecting mothers can also pass on protective antibodies to their newborns.
"This is a really great way to passively protect your baby because, unfortunately, this virus isn’t going away anytime soon," said senior author Dr. Emily Miller, assistant professor of obstetrics and gynecology at Northwestern University.
Researchers studied the blood of 27 pregnant women who’d received either the Pfizer or Moderna vaccine in their third trimester, as well as the umbilical cord blood of their 28 newborns.
RELATED: Babies can get COVID-19 antibodies from mothers while in the womb, study finds
They learned that women showed a robust immune response after vaccination, suggesting that the vaccine will protect pregnant women from COVID-19.
For most patients, more time between vaccination and delivery was associated with a more effective transfer of COVID antibodies to the baby.
Only three newborns did not have positive antibodies at birth and those mothers had received their first vaccine less than three weeks prior to their delivery dates.
Mothers who received the second vaccine dose before delivery also showed a higher likelihood of transferring antibodies to the child.
"This just gives extra fuel for people who are on the fence or just think, ‘Maybe I’ll wait until after I deliver,’" Miller said. "We strongly recommend you get the vaccine while pregnant. But if you’re fearing vaccination might harm the baby, these data tell us quite the opposite. The vaccine is a mechanism to protect your baby, and the sooner you get it, the better."
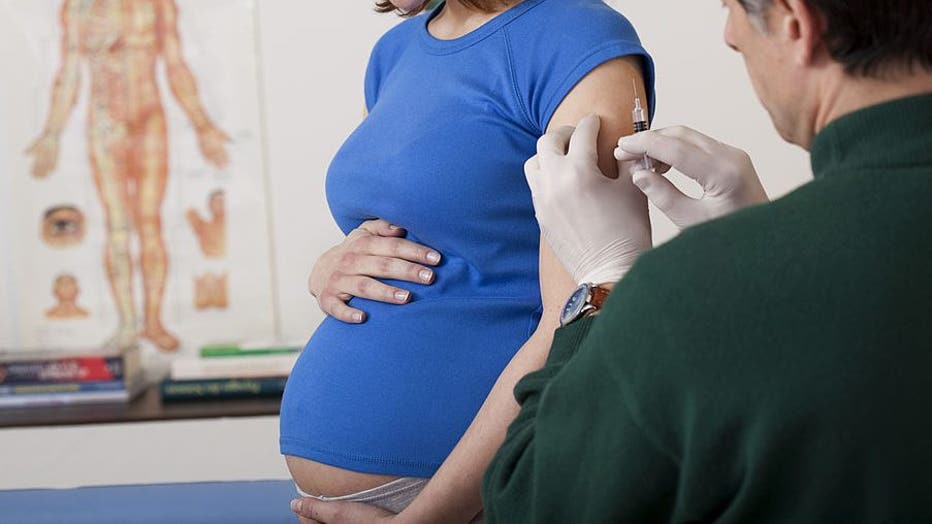
According to a new study, pregnant women who were vaccinated before delivery passed antibodies to their newborns. (Photo By BSIP/UIG Via Getty Images)
Since the vaccines have only been available since December, not enough time has passed to collect data on how first-trimester or second-trimester vaccinations would affect the mother and child. And it’s too soon to know how long such antibodies will last.
But Miller hypothesized it would lead to a more efficient antibody transfer.
Initial vaccine eligibility was limited to health care workers and the elderly, so most of the women who participated in the study work in the medical field.
The American Journal of Obstetrics and Gynecology published the study in its entirety on April 1.
This story was reported from Atlanta.

