FDA approves updated COVID-19 vaccines: Here’s what to know
COVID-19 vaccine for kids: CDC recommends adding shot to routine immunization schedule
A CDC panel COVID-19 shots should be added to the lists of recommended vaccinations for kids and adults. The panel's decision is not mandatory. State and local officials are allowed to make their decisions about vaccination requirements for school attendance. For example, flu and HPV shots aren’t required by many schools.
U.S. regulators approved updated COVID-19 vaccines on Thursday – shots designed to more closely target recent virus strains.
The clearance by the Food and Drug Administration means that Moderna and Pfizer can soon begin shipping out millions of doses. A third U.S. manufacturer, Novavax, expects its modified vaccine version to be available a little later.
The FDA approved Pfizer and Moderna’s updated Comirnaty and Spikevax COVID-19 vaccines, which target the omicron KP.2 variant, for individuals 12 years and older. Emergency use authorization for the vaccine was also granted for individuals 6 months through 11 years of age.
"We know that this virus is constantly changing, and that your protection from previous infection or from previous vaccines declines over time," James Mansi, the vice president of medical affairs at Moderna, said in a statement. "Ensuring that you receive the updated vaccine to better match what is circulating in the US will ensure that you are protected through the winter months when cases typically increase."
The agency's decision came a bit earlier than last year's rollout of updated COVID-19 vaccines.
When will new COVID-19 vaccine be available?
The drug companies said that the COVID-19 vaccines could be available within days.
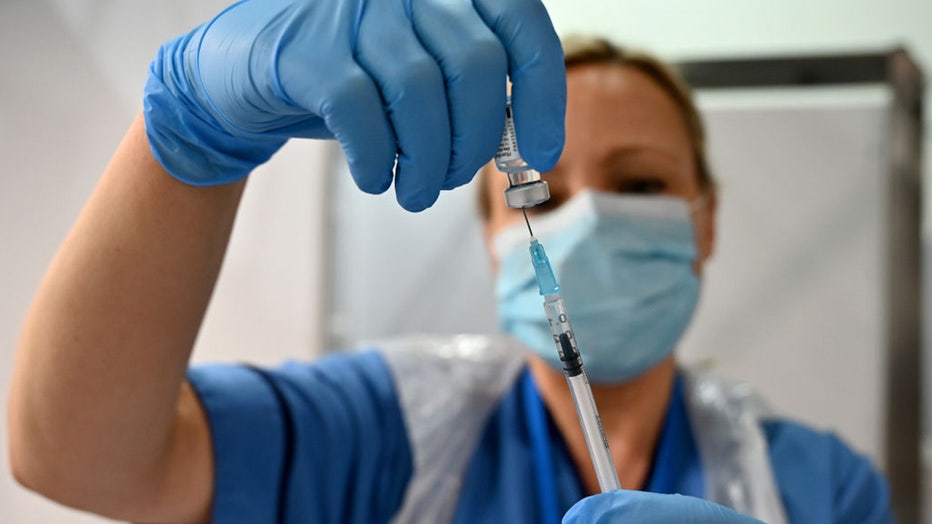
FILE - A health care worker prepares a COVID-19 vaccine at the Louisa Jordan Hospital as the roll out begins on Dec. 8, 2020 in Glasgow, Scotland. (Credit: Jeff J Mitchell - Pool /Getty Images)
Who should get updated COVID-19 vaccine?
Amid a summer wave of the virus across the country, the Centers for Disease Control and Prevention already has recommended this fall's shot for everyone aged 6 months and older.
While most Americans have some degree of immunity from prior infections or vaccinations or both, that protection wanes. CDC data shows only about 22.5% of adults and 14% of children received last fall’s shot.
RELATED: COVID-19 falls to 10th leading cause of death in US, health officials reveal
Skipping the new shot is "a hazardous way to go," because even if your last infection was mild, your next might be worse or leave you with long COVID symptoms, said Dr. Robert Hopkins Jr. of the National Foundation for Infectious Diseases.
When should I get updated COVID-19 vaccine?
People who are at high risk from the virus shouldn't wait but instead schedule vaccinations once shots are available in their area, Hopkins advised.
That includes older adults, people with weak immune systems or other serious medical problems, nursing home residents and pregnant women.
Healthy younger adults and children "can get vaccinated anytime. I don’t think there’s a real reason to wait," Hopkins said – although it’s OK to seek the shots in the fall, when plenty of doses will have arrived at pharmacies and doctor’s offices.
The exception: The CDC says anyone who recently had COVID-19 can wait three months after they recover before getting vaccinated, until immunity from that infection begins to wane.
Hopkins, who sees patients at the University of Arkansas for Medical Sciences, calls it vital for more youngsters to get vaccinated this year – especially with schools starting as coronavirus levels are high around the country.
"COVID does not kill many children, thank goodness, but it kills far more children than influenza does," Hopkins said, adding that teachers, too, should quickly get up to date with the vaccine.
Health authorities say it's fine to get a COVID-19 and flu vaccination at the same time, a convenience so people don't have to make two trips. But while many drugstores already are advertising flu shots, the prime time for that vaccination tends to be late September through October, just before flu typically starts its cold weather climb.

